advertisement

Editors Selection IGR 13-2
Response

Comment by Francesca Cordeiro & Li Guo on:
19654 Targeting amyloid-β in glaucoma treatment, Guo L; Salt TE; Luong V et al., Proceedings of the National Academy of Sciences of the United States of America, 2007; 104: 13444-13449
See also comment(s) by Robert N. Weinreb • Art Neufeld & Lan Wang & Chris Leung & James Morgan & Ian Trounce & Jonathan Crowston & Yeni Yucel & Balwantray Chauhan & Keith Martin •We are delighted to have our paper chosen as a topic for discussion in the brand new IGR section called 'Glaucoma Dialogue', and most appreciative that our work has generated so much interest.
We welcome the comments we have received, and the opportunity to respond to them. These comments fall into several broad categories, namely: IOP data; RGC counts; DARC imaging in vivo results; and previous amyloid-b and related studies; and we expand on each of these areas with our detailed responses below.
1. IOP data
Several comments have addressed the issue of IOP measurement data, which was felt to be lacking. We would like to emphasize that due to the strict limitation of words and space for PNAS, we were unable to present all the data accumulated from the various studies and these included our IOP data.
We did not have an opportunity to mention before that the IOP profiles were similar between the different treatment groups. IOP analysis from these studies showed that there was no significant difference in either mean peak IOP or integral IOP between any treatment and control groups - for example, the mean peak IOP in single agent treatment groups was 20.62 ± 3.73 (control), 21.34 ± 4.38 (Ab antibody), 17.16 ± 3.2 (Congo Red), and 16.36 ± 3.98 (β-secretase inhibitor) mmHg.
The actual method of raising the IOP using the Morrison hypertonic saline episcleral injection has been well-established in our group with consistent and reproducible results (Cordeiro, Guo et al. 2004; Guo, Moss et al. 2005; Guo, Tsatourian et al. 2005; Guo, Salt et al. 2006; Guo, Salt et al. 2007). For the record, we do indeed use an episcleral ring during the procedure as John Morrison has well described (Morrison, Moore et al. 1997).
2. RGC and RGC apoptosis counts
Several experts have commented that there were insufficient data regarding absolute RGC numbers. Again we would emphasize that we could not show all our data due to strict PNAS restrictions in manuscript length. In addition, in summarizing the data we also had to allow for the comparison between our other publications (Cordeiro, Guo et al. 2004; Guo, Salt et al. 2006), where % reductions and ratios of absolute counts were used to compare effects of treatment or time, e.g., Figure 6G shown below.
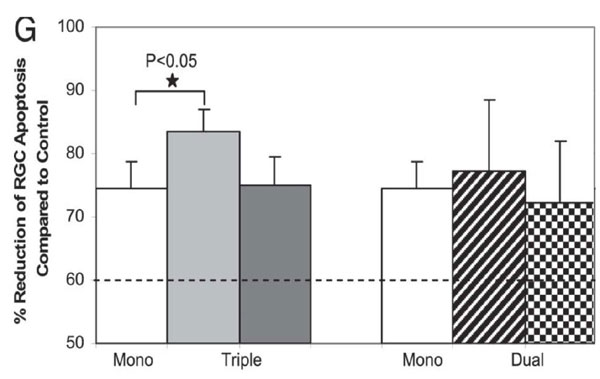
Fig. 6.Effect of combining agents targeting Aβ in glaucoma.In vivo DARC images show the effects of triple (C and D) and dual (E and F) therapies on prevention of RGC apoptosis at 3 weeks after IOP elevation compared with control (A) and Abab monotherapy (B). (G) Triple therapy (triple Abab) significantly reduced RGC apoptosis compared with Ababalone (*,P-0.05). In fact, the triple therapy resulted in 84% mean reduction of RGC apoptosis compared with 74% by Abab monotherapy. All other combining therapies showed significant reduction of RGC apoptosis compared with control, although there was no statistic significance compared with Abab monotherapy. For comparison, the dashed line represents the results of our previous study, where we had combined two different glutamate modulators (MK801 and an mGlut agonist) (22) and shown a 60% reduction of RGC apoptosis at 3 weeks after IOP elevation. (Guo et al. PNAS 2007; 104: 13447)
There is a query regarding the presence of RGC apoptosis in normal control eyes. This and all our previous studies confirm that there is baseline level of RGC apoptosis in normal eyes (Cordeiro, Guo et al. 2004; Guo, Moss et al. 2005; Guo, Salt et al. 2006). In our present study we had, as before, a baseline mean level of apoptosis, which in absolute terms was 324.50 (see Fig. 2B).
To allow us to ascertain absolute RGC counts, we performed Dil retrograde labelling, as described in the methods section of the paper, in a small number of animals at all time points. These animals had absolute RGC counts done from whole retinal flat mounts. The absolute mean RGC number in untreated control OHT animals was consistent with our previous data (Cordeiro, Guoet al. 2004). From the total RGC number we were then able to calculate the ratio of the apoptotic RGC count to the absolute RGC count. This is shown in Figure 5B as a percentage.
In Figure 5C, we compared all groups with IOP elevation untreated (non antibody) control to get a percentage of RGC apoptosis. In OHT untreated eyes at 3 weeks the mean absolute number of apoptotic RGC is 3458.50. In control non-OHT eyes it is 324.50. Hence we calculated this as a 9.38%RGC apoptosis compared to control, as displayed in Figure 5C.
Below we show a comparison of the published % reduction graph (Fig. 5C) with the graph with corresponding absolute numbers, as requested by some experts for clarification.
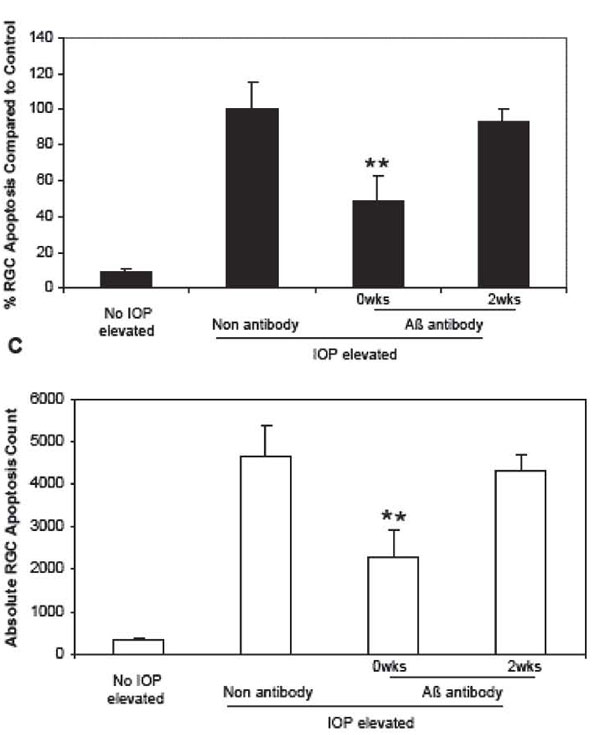
Fig. 5. C.Corresponding absolute RCG apoptosis counts. (Guo et al. PNAS 2007; 104: 13447)
3. DARC imaging
The DARC imaging technique has been described in several publications by our group since 2004, which perhaps are not widely known in the field of glaucoma:
- Cordeiro MF, Guo L, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA 2004; 101: 13352-13356.
- Guo L, Moss SE, et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. IOVS 2005; 46: 175-182.
- Guo L, Salt TE, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo.IOVS 2006; 47: 626-633.
- Maass A, Von Leithner PL, et al. Assessment of Rat and Mouse RGC Apoptosis Imaging in Vivo with Different Scanning Laser Ophthalmoscopes. Curr Eye Res 2007; 32: 851-861.
- Guo L, Salt TE, et al. Targeting amyloid-β in glaucoma treatment. Proc Natl Acad Sci USA 2007; 104: 13444-13449.
In this paper (Guo, Salt et al. 2007) and our most recent paper (Maass, von Leithner et al. 2007), we have used in vivo RGC apoptosis counts for assessment of treatments, as we believed the validation of the DARC counts from histology in the previous papers itemised above (1-3), had already shown the value of the technique, and the use of annexin as a label for in vivo RGC apoptosis confirmed by other groups (Reichstein, Ren, et al. 2007).
As we have previously highlighted, one of the advantages of DARC technology is that it allows longitudinal study of the same animal over time.
In illustrating the effects of treatments with in vivo DARC imaging, as in Figure 4, serial images from the same animals were used,i.e., images from treatment with the β-secretase inhibitor (J (3 weeks), K (8 weeks) and L (16 weeks)), Congo Red (G (3 weeks), H (8 weeks) and I (16 weeks)) and Aβ antibody (D (3 weeks) and E (8 weeks)) - save for image F (Aβ antibody,16 weeks), where the imaging quality at 16 weeks in this animal was not adequate for publication purposes.
One comment received was regarding the risk of repeated intra-vitreal injections causing cataract, vitreous hemorrhage, or retinal detachment leading to media opacity and imaging difficulties.
For clarification, all our treatments in these studies were single applications. OHT animals were repeatedly imaged with DARC however three times over 16 weeks, but cataract, vitreous haemorrhage, or retinal detachment were not encountered. However there was some incidence of corneal epitheliopathy which appeared related to the length of time animals were under anaesthesia with insufficient corneal lubrication.
Several comments have been made as to whether the apoptosis is only confined to RGCs in our glaucoma-related models using DARC. This has been a question that we have attempted to address in all five of the peer-reviewed publications given above (Cordeiro, Guo et al. 2004; Guo, Moss et al. 2005; Guo, Tsatourian et al. 2005; Guo, Salt et al. 2006; Maass, Von Leithner et al. 2007). Our histological data, in this study and our previous studies, have clearly co-localized apoptosis to retrogradely labelled
RGCs in whole retinal mounts and also in retinal cross-sections (Cordeiro, Guo et al. 2004; Guo, Moss et al. 2005; Guo, Tsatourian et al. 2005; Guo, Salt et al. 2006; Maass, Von Leithner et al. 2007). We have not specifically looked at amacrine or microglial cells however. Again, we have previously confirmed using anti-caspase3 immunostaining (Cordeiro, Guo et al. 2004) that annexin 5 positivity is a direct correlate of apoptosis, and other groups have confirmed this finding too (Reichstein, Ren et al. 2007).
There were some comments implying that we used the fellow eyes in treated animals as controls. This is categorically not the case, as control eyes were OHT eyes (animals ethically could only have one eye with elevated IOP) which had vehicle or null antibody treatment, and this is clearly stated in our paper.
4. Previous Amyloid-β and related studies
Our paper provided a limited view of previous studies, again because of the PNAS space restrictions. Nevertheless, we referenced papers by McKinnon, Lehman et al. 2002; Loffler, Edward et al. 1995; Vickers, Lazzarini et al. 1995; Blanks, Schmidt et al. 1996; Blanks, Torigoe et al. 1996; Archer, Hirano et al. 1998; Parisi, Restucciaet al. 2001; Bayer, Keller et al. 2002; Johnson, Leitneret al. 2002; Wostyn, 2004; to cover some publications suggesting evidence of similar mechanisms in the brain and in the retina.
We regret we were unable to quote all papers in the area, particularly the more recent ones providing even more evidence of a possible relationship, including the following, and we would urge readers interested in this area to refer to these recently published papers for additional information, the findings of which we have briefly summarised here.
- Demonstration of retinal abnormalities including RNFL loss and retinal vascular changes in early AD (Berisha, Feke et al. 2007)
- A reduction in the number of ON fibers in patients with AD, with a threefold greater odds ratio for a larger optic cup-to-disc ratio in patients with AD (Danesh-Meyer, Birch et al. 2006)
- Evidence of reduced cerebral blood flow in AD and NTG patients, (Sugiyama, Utsunomiya et al. 2006)
- Heilicobacter pylori implicated as a possible underlying factor in AD and NTG (Kountouras, Zavos et al. 2007)
- Suggestion that abnormal APP processing may be involved in RGC loss in DBA/2J glaucoma mouse model (Goldblum, Kipfer-Kauer et al. 2007)
- A review of mechanisms in glaucoma being similar to other neurodegenerative diseases (Gupta and Yücel 2007)
- A high frequency of open-angle glaucoma in AD patients (23.8%) compared to controls (9.9%) in a Japanese study (Tamura, Kawakami et al. 2006)
As we mention in our paper, there has been other work, especially that of Walsh, Jens et al., looking at the effects of Aβ in the retina. In their study, as we comment on in our paper, apoptosis was induced in photoreceptor and inner nuclear cells, although they noted 'progressive loss or atrophy of ganglion cells over a long period of time' (Walsh, Monteroet al. 2002; Walsh, Bresciani et al. 2005). Several differences exist between our study and theirs: firstly, the dose they applied was much greater (2-5 nmol compared to 0.55 nmol); secondly, their study was up to five months after intravitreal exposure of Aβ; thirdly, they used TUNEL labeling on histological specimens as opposed to the in vivo demonstration of annexin-positive cells in our study; and finally, they used Sprague-Dawley rats.
We found no evidence of extensive RGC loss in retrogradely labelled RGCs on whole retinal mounts
One expert has suggested that the treatments targeting Aβ may themselves have been neurotoxic, especially when combined, and this resulted in such extensive RGC loss that annexin 5 labelling was reduced, and optic nerve cupping produced (pointed out apparently in Fig. 5F). We found no evidence of extensive RGC loss in retrogradely labelled RGCs on whole retinal mounts. Also, we do not agree that there was cupping, which anyway is a controversial finding in the rat ONH.
Although there is no study looking at Aβ deposition in post mortem glaucoma eyes, we do refer in our paper to the one human study of measurements of ocular Aβ in the patients - this showed that there was a decrease in vitreous Aβ which was felt by the authors to be 'consistent with retinal Aβ deposition' in patients with glaucoma (Yoneda, Hara et al. 2005).
Further work is needed to establish the role of amyloid-β and abnormal APP processing in glaucoma patients
Our studies do suggest that Aβ 'is a likely mediator of pressure-induced RGC death…' and there is increasing evidence from other groups supporting this view. However, we strongly believe that further work is needed to establish the role of amyloid-β and abnormal APP processing in glaucoma patients.
But of much more importance, this work highlights a new and potential route of treatment in glaucoma.
Comments
The comment section on the IGR website is restricted to WGA#One members only. Please log-in through your WGA#One account to continue.Log-in through WGA#One
