advertisement

Editors Selection IGR 13-4
Comment

Comment by Clement Tham on:
70327 Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial, Azuara-Blanco A; Burr J; Ramsay C et al., Lancet, 2016; 388: 1389-1397
See also comment(s) by Rupert Bourne • Benjamin Xu • Jennifer Burr & Augusto Azuara Blanco •The EAGLE study is a landmark randomized controlled trial (RCT) that directly compared 'clear lens' extraction and laser peripheral iridotomy in newly-diagnosed eyes with primary angle closure (PAC) or primary angle-closure glaucoma (PACG). The co-primary endpoints were patient-reported health status, intraocular pressure, and incremental cost-effectiveness ratio per quality-adjusted life-year gained 36 months after treatment. The authors concluded that clear lens extraction showed greater efficacy and was more cost-effective than laser peripheral iridotomy.
Prior to the EAGLE study, earlier RCTs examined the role of lens extraction alone in established cases of PACG with prior laser peripheral iridotomy, in eyes with visually significant cataract,1-3 and eyes without.4 Another RCT evaluated the role of lens extraction in eyes with acute primary angle closure (APAC), after the initial intraocular pressure (IOP) control, versus laser peripheral iridotomy.5 The EAGLE study focused specifically on the subgroup of newly-diagnosed nonacute PAC and PACG eyes, without prior laser peripheral iridotomy, and compared clear lens extraction against iridotomy. The EAGLE study focused on the very first step in the management algorithm of this subgroup of eyes, and filled an important knowledge gap in the PAC / PACG literature.
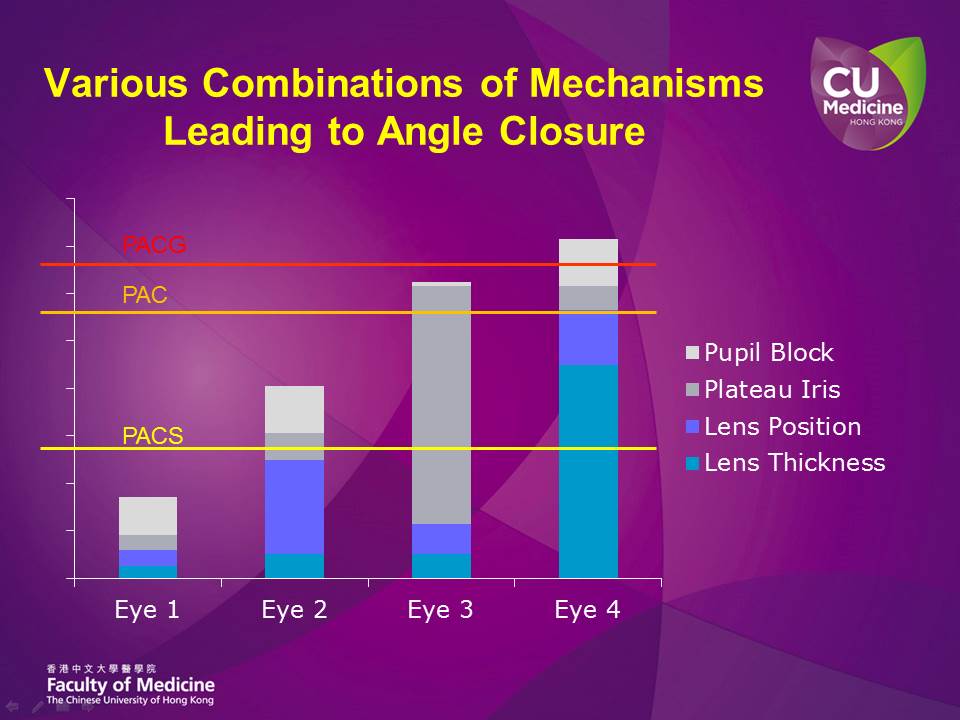
While all these trials are important in comparing lens extraction against other 'conventional' or 'standard' treatment options in specific angle closure scenarios, they do all share one critical shortcoming in their study design: that the mechanisms leading to angle closure were not considered, and the PAC or PACG eyes were all grouped together as one single entity for study evaluations. Today, we are aware of the various mechanisms that predispose an anterior chamber drainage angle to narrowness and closure. The more common and important mechanisms would include pupillary block, plateau iris configuration, and the lens position and/or thickness. In each eye, these mechanisms could be of quite different relative importance (Fig. 1). For example in Eye 1 in Figure 1, pupillary block may play the most important role amongst these mechanisms, but the overall predisposition to angle closure (reflected by the height of the Eye 1 column) is not high. The lens position plays the most crucial role in Eye 2, while plateau iris may be the most important mechanism in Eye 3. In Eye 4, the lens (both position and thickness) is playing a dominant role in causing angle closure. Conceptually, the correct management approach in eyes with PAC/PACG should be to first identify the relative importance of these angle-closing mechanisms, and then select the intervention (or combination of interventions), with the least risks, that allows us to reduce the height of the 'risk column' (Fig. 1) by the greatest amount, and thereby safely and significantly eliminating the predisposition to angle closure in that particular eye. In Eye 3, where plateau iris is the predominant mechanism, lens extraction or iridotomy may not be effective. On the other hand, if Eye 4 with predominantly lens mechanism causing angle closure is randomized in a trial to receive iridotomy alone as primary treatment, the laser may not appear effective too. In situations where extensive peripheral anterior synechiae (PAS) is present, we may of course have to consider additional IOP-lowering procedures, such as goniosynechialysis or filtration surgery, in addition to procedures that reverse the predisposition to angle closure to help reach a safe target IOP.
Fig 1: Fig 1: Flowchart summarizing Pathophysiology of PDS and PG.
At present, there are still gaps in our knowledge that may prevent us from adopting this logical approach in every PAC/PACG eye. Future studies may help us identify anatomical parameters, from gonioscopy or imaging, that could allow us to determine the relative contributions of such mechanisms, and to help us identify and most effective and safest approach in each eye.
References
- Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology 2008;115(12):2167-2173 e2162.
- Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification vs phacotrabeculectomy in chronic angle-closure glaucoma with cataract: complications [corrected]. Arch Ophthalmol 2010;128(3):303-311.
- Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology 2009;116(4):725-731, 731 e721-723.
- Tham CC, Kwong YY, Baig N, Leung DY, Li FC, Lam DS. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology 2013;120(1):62-67.
- Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology 2008;115(7):1134-1140.
Comments
The comment section on the IGR website is restricted to WGA#One members only. Please log-in through your WGA#One account to continue.Log-in through WGA#One