advertisement

Editors Selection IGR 24-1/2
Response


Comment by David Sinclair & Bruce Ksander on:
111960 Sustained Vision Recovery by OSK Gene Therapy in a Mouse Model of Glaucoma, Karg MM; Lu YR; Refaian N et al., Cellular reprogramming , 2023; 25: 288-299
See also comment(s) by Harry Quigley • Derek Welsbie • Pete Williams •We thank the editors for selecting our two papers for discussion at IGR. The first paper (Y Lu et al. Nature, December 2020) was discussed in a previous IGR issue and the second (M Karg et al. Cellular Reprogramming, 2023;25(6)) is discussed in the current issue. Since we did not have the opportunity to respond to the previous comments regarding our Nature paper, and some of these issues were raised again, here we will discuss both papers.
Papers published in scientific journals are supposed to be rigorously reviewed by our peers and experts in the field. However, this process has historically not been available for public scrutiny. The journal Nature and some other journals have recently adopted a new policy in which the reviewers are anonymous during the review process, but after a paper is accepted for publication, the reviewers can consent to identifying themselves and publishing their review comments and the authors can consent to publishing their responses. For our study in Nature, the reviewers and all authors consented and a complete copy of the 53-page peer review was published and can be accessed on-line ( Peer Review file). The second paper published in Cellular Reprogramming does not publish peer review comments. Because readers of any scientific study will inevitably have their own questions or clarifications, we welcome the opportunity to answer questions raised by Dr. Harry A. Quigley, Dr. Derek Welsbie, and Dr. Pete A. Williams.
The discussants comments, identified by their initials HAQ, DW, and PAW), and our responses are below.
(DW) The idea that OSK expression can be used to reverse aging and restore vision is very enticing. However, it is not clear that there has been a single, rigorous demonstration of this concept. The field desperately needs independent validation using appropriate controls.
(PAW) Researchers and ophthalmologists should study and question these findings with a rigorous eye… Rigorous testing and confirmation of findings by other research groups should be the first step towards this.
We completely agree with the need for rigorous testing and confirmation of all scientific studies, which is always an on-going process. We have attempted here to briefly summarize the current status of in vivo epigenetic reprogramming to reverse aging and rejuvenate cells, which is a rapidly growing field. Following our 2020 report in Nature, demonstrating reversal of physiological aging and the reestablishment of youthful DNA methylation and mRNA patterns, along with a decrease in epigenetic age and the rejuvenation of retinal ganglion cell function, multiple laboratories published studies in 2022 that reproduced age-reversal effects in a variety of other non-ocular tissues. For example, Juan Carlos Belmonte and coworkers reported age reversal in vivo using transient expression of OSKM reprogramming genes that reversed the epigenetic clock and restored function to kidney and skin cells as measured by transcriptome and metabolome changes.1 Manuel Serrano's laboratory reported that in vivo reprogramming drove epigenetic transcriptome and metabolic changes towards a younger phenotype with increased function in the pancreas, liver, spleen, and blood.2 Wolf Reik also reported similar results in the skin.3 These studies did not use the identical OSK reprogramming construct we used in our experiments but rather OSKM, or a combination of reprogramming factors. We believe the fact that a variety of factors can be used to reverse epigenetic aging and rejuvenate cellular function indicates the robustness of this approach. The three 2022 papers were followed by many more publications,4‐13 including studies using chemicals to reverse aspects of aging in cells,14 and reviews.15,16 Together, these studies indicate that in vivo epigenetic reprogramming to reverse age and restore cellular function is possible in a wide range of different cell types and organs. The recent publication (in press) of lifespan extension on very old mice adds further weight to the body of evidence in favor of age reversal.17
However, I believe the question raised by Drs. Welsbie and Williams was whether anyone has specifically reproduced in vivo epigenetic reprogramming that reverses aging and restores visual function. While the answer to this question is "No, not yet," there is progress in this regard. Two papers currently under peer review and published on bioRxiv 18,19 both support our results by showing that in vivo reprogramming prevents retinal ganglion cell loss, and one also shows restoration of visual function.
Sienna Drake et al., from the Department of Neurology and Neuroscience at McGill University in Canada, report that the OSK reprogramming construct used in our study is capable of preventing RGC loss in experimental autoimmune encephalomyelitis (EAE), a model system for multiple sclerosis, though whether aspects of aging are reversed in this model is not yet clear.
Separately, W.L. Tai et al. from the Department of Ophthalmology at Harvard (not connected to Dr. Ksander's laboratory) used an epigenetic "switch" to target the DNA methyltransferase 3a (DNMT3a) to reprogram retinal ganglion cells and prevent neuronal loss, trigger axon regeneration, and increase visual function following an optic nerve injury. While this study did not show epigenetic age reversal, their data indicates that altering the epigenetic landscape of types of retinal ganglion cells is capable of reprogramming and rejuvenating ganglion cells, increasing their survival, and improving visual function. Since both papers are under peer review, we will have to wait to see the final versions of these studies, if and when they are published.
(HAQ) Only treated and saline-injected eye vision was measured, not fellow eyes, or bilateral untreated controls.
(DW) …the fundamental problem ‐ there was no control OMR group (i.e. injury without OSK expression).
We do not use contralateral or fellow eyes as controls because of the data indicating that contralateral eyes from the microbead-induced glaucoma model show evidence of microglia activation and therefore these eyes are not equivalent to an untreated eye.20,21 We use the eyes of mice that receive a unilateral injection of saline, which controls for the injection procedure. In addition to this saline control, we also have untreated control eyes. All mice had baseline OMR measurements taken before the experiment started. Therefore, we have both saline-injected control eyes as well as untreated control eyes.
"…there was no control OMR group (i.e. injury without OSK expression)." The injury without OSK expression control was included (see figure 1d). Mice received a microbead injection followed by AAV2-OSK injection on day 29 but they did not receive any Dox treatment. These mice have the injury and RGCs are transduced with AAV2-OSK, but the OSK gene is not turned on by Dox. This is the best control for comparing injury with OSK treatment to injury without OSK treatment because it controls for the possible effects of AAV2 transduction on retinal ganglion cells without expression of OSK.
|
Untreated control group- |
baseline OMR measurements on all mice before the experiment started |
|
Grp 1. Saline (no glaucoma) + no treatment |
Negative control; no glaucoma |
|
Grp 2. Glaucoma (beads injection) + AAV2-OSK + no Dox |
Neg. control for OSK treatment; AAV control |
|
Grp 3. Glaucoma (beads injection) + AAV2-OSK + Dox |
Experimental group- inducible OSK |
|
Grp 4. Glaucoma (beads injection) + AAV2-OSK (always on) |
Experimental group- (non-inducible) OSK |
The OMR and pERG visual function assays require a clear cornea. The exclusion of mice with edematous corneas did not alter, either up or down, the overall IOP levels of the mice receiving microbeads. The IOP levels in the Nature paper and the Cellular Reprogramming paper were consistently within the same range. We learned the magnetic microbead injection method from Dr. Adriana Di Polo and have visited her lab several times to perfect the technique. Our results are consistent with her results on the loss of retinal ganglion cells and the level of IOPs her lab reports.
Ito, Y. A., Belforte, N., Vargas, J. L. C. & Polo, A. D. A Magnetic Microbead Occlusion Model to Induce Ocular Hypertension-Dependent Glaucoma in Mice. J Vis Exp Jove 53731 (2016). doi:10.3791/53731
(DW) OMR is a subjective test in which the investigator determines whether or not a head movement "counts".
As stated in the methods sections of our papers, we quantified OMR with a Striatech Optodrum instrument, which uses a computer driven algorithm combined with a video monitoring system that captures the outline of the mouse, while nose and tail pointers are used to track head movement. The computer has an algorithm for identifying positive and negative tracking behavior of the mice. All head movements are video recorded, and two lab members independently confirm all measurements identified as positive or negative by the computer, without knowing which groups the mice are from. The computer also has an algorithm for progression of the cycles / degree. This instrument has been available for many years and has been used in many published papers. The Striatech website ( https:// stria.tech/optodrum/) and support staff can provide more information on their computer-controlled system. Thus, our OMR method is not subjective.
(PAW) How does the addition or removal of methyl groups benefit the aging retinal ganglion cells and how does this provide a protection of visual function under stress?
(PAW) What is the mechanism of visual recovery following epigenetic reprogramming?
We are at the earliest stages of understanding the mechanism of OSK-mediated in vivo epigenetic reprogramming. From our studies reported in Nature, we know that OSK treatment of retinal ganglion cells following an optic nerve crush injury is dependent on CpG demethylation mediated by Tet1 and Tet2, as well as TDG (thymine DNA glycosylase) (see Fig. 2). Moreover, OSK-mediated treatment of retinal ganglion cells that reversed physiological aging and restored OMR visual function was also dependent on a Tet1- and Tet2- mediated mechanism (see Fig. 4, and Extended Data Fig. 10) and the methylation sites implicate an involvement of the PRC2 complex (polycomb repressive complex 2) (see Extended Data Fig. 10). From these results, we proposed a mechanism for OSK-mediated epigenetic reprogramming of retinal ganglion cells (see Extended Data Fig. 10d), which has been expanded in Lu et al., 2024 (Nature Aging).
We have yet to demonstrate whether Tet1, Tet2, TDG, and members of the PRC2 complex, or other epigenetic modulators and transcription factors are involved in the restoration of visual function by OSK treatment of retinal ganglion cells in the glaucoma mouse model. This will be studied in future experiments.
(PAW) Is epigenetic reprogramming causing a transient or sustained increase in RGC function that is fully recognized and utilized by the rest of the visual system? Or is this just a function of the power and relatively low n in these groups?
The effect of epigenetic reprogramming on the visual function of RGCs is fully recognized by the visual system within the limitations of OMR visual acuity assessment. The OMR long-term assessment had n= 10 mice for beads with continuous OSK, n=10 mice for the saline control group, and n=8 mice for the beads with cyclic OSK group.
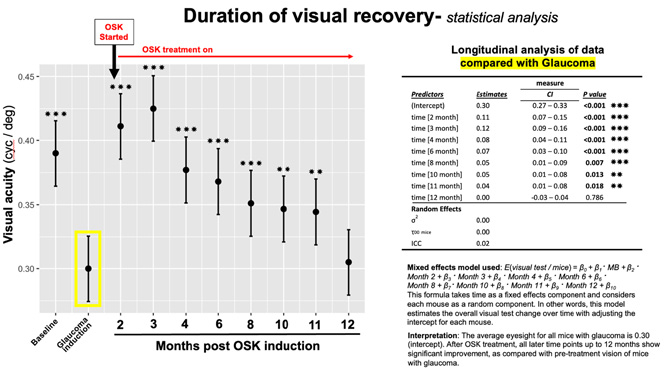
In these studies, we made serial OMR reading of the same mice at baseline, after microbead treatment for 4 weeks, when elevated pressure induce loss of visual function was detected, and again after AAV2-OSK treatment at intervals over the next 12 months. The statistical analysis was performed by the biostatistician at the core facility at the Mass Eye & Ear, who used a mixed effects model, which is described in a different format in the figure below in an attempt to answer this question more clearly. This statistical analysis ruled out the possibility these results were "just a function of the power and relatively low n in these groups."
(PAW) No comment on other vital metrics of RGC health and recovery- synaptic and dendritic remodeling, soma counts, axon counts in the optic nerve.
This study had a very narrow focus on the duration of changes in visual function as measured by OMR. A more expansive analysis of these other important and vital measurements is in progress or planned for future experiments- synaptic and dendritic remodeling, soma counts, and long-term axon counts in the optic nerve
(PAW) There is no age-related structural or visual loss at the 21-month time point.
In our Cellular Reprogramming paper Figure 4, we used OCT to determine the retinal thickness of 21-month-old mice in which AAV2-OSK was expressed continuously and compared them with: uninjected, saline treated, and glaucoma (bead injected) mice treated with cyclic-OSK. Since this experiment was done to look for evidence of tumor formation in the retina, we did not compare this data with the retinal thickness of young mice. Age-related thinning of the retina in mice has been reported by several groups.22,23
We previously studied the effect of epigenetic reprograming on retinal aging (see Nature paper) where we reported that 12-month-old mice had reduced visual function as measured by pERG and OMR and this was reversed by OSK reprogramming (additional studies on old mice are summarized below).
(PAW) These experiments were performed on young, female mice. As these are young mice… it is not representative of the patient population that would be aged…
We completely agree with this comment. There are at least two important components of glaucoma (i) stress induced RGC injury by elevated IOP, and (ii) the negative effects of aging that increases RGC susceptibility to stress. Therefore, the only way we can completely understand the pathobiology of glaucoma is to use model systems that include the effects of both aging, and stress (elevated intraocular pressure). These experiments are underway.
We did demonstrate that OSK-mediated epigenetic reprogramming of retinal ganglion cells does reverse age and restore visual function in old mice (12-months-old) as reported in the Nature paper. We currently know more about the effects of reprogramming on old mice than we do about reprogramming young mice with glaucoma. Our results for reprogramming retinal ganglion cells in aging mice from our study are summarized below and the corresponding figures in the paper are cited.
OSK epigenetic reprogramming reversed physiological aging
- Decreased visual function by pERG and OMR in aged mice (12 months old) (Fig. 4).
- OSK treatment for 4 wks significantly restored visual function in 12-month-old mice (Fig. 4, Sup Fig. 9)
- The age-related change in the retinal ganglion cell transcriptome as measured by bulk RNAseq of enriched retinal ganglion cells was returned to a more youthful pattern by OSK treatment (Fig. 4, Sup Fig. 9).
- There was an age-related change in the retinal ganglion cell methylome that was restored to a more youthful pattern by OSK treatment (Sup Fig. 10).
- There was a decrease in the epigenetic age of OSK-treated retinal ganglion cells in old mice (Fig. 4, Sup Fig. 10).
- OSK reprogramming that restored OMR function in old mice was Tet1- and Tet2- dependent (Fig. 4, Sup Fig. 10).
- OSK reprogramming of retinal ganglion cells targeted components of the PRC2 complex (polycomb repressive complex 2) (Sup Fig. 10).
(PAW) The AAV2 vector used in these experiments is not retinal ganglion cell specific, so there is likely to be effects that have not been fully assessed in other retinal cell types.
There is an extensive literature indicating AAV2 targets retinal ganglion cells in mice, non-human primates, and has been used in clinical trials to target the retinal ganglion cells of patients with LHON (Leber Hereditary Optic Neuropathy).24‐26 We have also shown in our 2020 Nature paper that our AAV2 vector predominantly targets retinal ganglion cells (see Figure 1 and Extended data Fig. 2 a, b). There is evidence that AAV2 can target a low percentage of Muller glial cells.27 Thus, I believe Dr. Williams question is important regarding how we can be sure that the effects of reprogramming are retinal ganglion cell specific and cell autonomous and this was a topic extensively discussed in the review of our paper (see Peer Review file). With these questions in mind, we expressed OSK in amacrine cells, using as transgenic mouse system, and demonstrated this had no effect on ganglion cell survival or axon regeneration following optic nerve crush (see Extended Data Fig. 4). Future work should include this type of experiment using the glaucoma model system.
(PAW) A study by Yu Wai Man et al. demonstrated that AAV2 is promiscuous and was found throughout the visual tract of the untreated (uninfected) contralateral eye in a non-human primate.
This is true but it doesn't affect our paper's conclusions. The paper by Yu Wai Man demonstrates that an intravitreal injection of AAV2 in one eye of a non-human primate results in the vector being detected in the contralateral eye at low levels. They detected the vector in the anterior segment, the retina (whole retina; no cell types defined), and optic nerve. They detected the vector "below the level of quantification (250 copies /mg of DNA) in the optic chiasm, optic tract, and lateral geniculate nucleus. They performed this experiment because they were trying to explain a positive improvement in the visual function of the untreated contralateral eye in patients with LHON (Leber Hereditary Optic Neuropathy) that were treated with their gene therapy (AAV2-ND4). We agree the data from this study supports the authors conclusions that "a bilateral effect of unilateral intravitreal injection [of AAV2] targeting retinal ganglion cells suggests that interocular diffusion of viral DNA vector could occur." They also state that further investigations are needed to confirm this. We agree this an important study but would note that this did not stop them from subsequently conducting a phase 3 clinical trial of their gene therapy that they claimed targets the retinal ganglion cells.
Yu-Wai-Man, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med 12, eaaz7423 (2020).
(PAW) … future work with have to assess the whole mouse for any potential tumors or metastases.
While we found no evidence of tumor formation within the retina in mice treated with AAV-OSK for 21 months, we agree that a complete ocular and systemic safety profile following intravitreal injection of AAV2-OSK would be informative.
(HAQ) There is no data indicating conclusively that neuronal function is retained in the long-term and that survival of RGCs is enhanced. The outcomes are based solely on the OMR response.
(HAQ) There was no data provided that vision was lost only that a temporary reduction was reversed. Evidence in both papers.
(HAQ) Was there RGC loss at all in either treated or untreated groups.
Data showing the effects of microbead induced elevated intraocular pressure are not temporary are in Supplemental figure 2. This study was primarily focused on looking at the duration of changes in visual function as measured by OMR following OSK epigenetic reprogramming and, now that we know the duration of this effect, we are planing additional studies that will measure more RGC function and structure at specific time points.
(HAQ) Methodological issues in the Nature paper- Axon density data are a poor method to assess survival of RGC axons... Instead, density times nerve area provides definitive axon counts.
The area of optic nerve sections used to determine the axon density were measured and there was no significant difference between the groups. Similarly, when the data was graphed as total axon counts, this did not change the results.
(DW) Amazingly, with both Tet-on and tet-off strategies, there was a rapid reversal of vision loss, claimed to be even better than the initial baseline. Unfortunately, extraordinary claims require extra ordinary data and the study here was plagued by major omissions and inconsistencies.
(PAW) How is it possible that improved visual acuity was significantly better than the baseline level of vision before the experiment started.
(HAQ) No explanation is offered for how vision in treated eyes would be better than normal.
We agree with the comments that this effect is unexpected, even more so when we considered that there was no increase in visual function when OSK reprogramming of retinal ganglion cells was performed in young (4-month-old) healthy mice (Nature paper, Extended Data Figure 9 e, g, h). We have yet to determine a mechanism that could mediate this effect. However, if we were to speculate on how this is possible, one explanation is based on the recent finding that RGC injury accelerates aging.28,29 If so, perhaps epigenetic reprogramming has a greater rejuvenating effect on young injured RGCs as compared with young healthy RGCs because they are epigenetically older. This rejuvenating effect could possibly occur by increasing sprouting of axons or increasing synaptic connections. However, this is just an hypothesis.
(DW) The authors did use a complementary approach to measure RGC function, pERG but unexplainably, there was no experimental pERG group (injury with OSK expression).
(HAQ) No pERG data is presented to show that pERG was improved.
We routinely measure pERG and OMR on mice, as indicated. However, at the beginning of this long-term study, the pERG instrument broke and took a while to be replaced. This is the reason for the lack of pERG measurements in this long-term study. In the supplementary data section, we did include the pERG results from a separate study of wild-type mice treated with a control AAV2-vector to demonstrate that pERG amplitude was lost at 3 wks after microbead injection with no restoration observed out to 27 wks. Thus, demonstrating in our microbead-induced model of glaucoma, loss of RGC function was not transient but sustained.
References
- Browder KC, Reddy P, Yamamoto M, et al. In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat Aging. 2022; 2(3):243-253. doi:10.1038/s43587-022-00183-2
- Chondronasiou D, Gill D, Mosteiro L, et al. Multi‐omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell 2022 Mar;21(3):e13578. doi:10.1111/acel.13578
- Gill D, Parry A, Santos F, et al. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife. 2022:11:e71624.
- Chen Y, Lüttmann FF, Schoger E, et al. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science. 2021;373(6562):1537-1540.
- Farber G, Liu J, Qian L. OSKM-mediated reversible reprogramming of cardiomyocytes regenerates injured myocardium. Cell Regen. 2022;11(1):6.
- Mitchell W, Goeminne LJE, Tyshkovskiy A, et al. Multi-omics characterization of partial chemical reprogramming reveals evidence of cell rejuvenation. 2023:2023.06.30.546730. doi:10.7554/elife.90579.2
- Kriukov D, Khrameeva EE, Gladyshev VN, Dmitriev SE, Tyshkovskiy A. Longevity and rejuvenation effects of cell reprogramming are decoupled from loss of somatic identity. bioRxiv. 2022: 2022.12.12.520058. doi:10.1101/2022.12.12.520058
- Yang J-H, Hayano M, Griffin PTet al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2024;187(5):1312-1313. doi: 10.1016/j.cell.2024.01.049
- Schoenfeldt L, Paine PT, M NHK, Phelps GB, Mrabti C, Perez K, Ocampo A. Chemical reprogramming ameliorates cellular hallmarks of aging and extends lifespan. Biorxiv. 2022:2022.08.29.505222. doi:10.1101/2022.08.29.505222
- Wang C, Ros RR, Martinez-Redondo P, Ma, et al. In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nat Commun. 2021;12(1):3094.
- Olova N, Simpson DJ, Marioni RE, Chandra T. Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell. 2019;18(1):e12877.
- Sarkar TJ, Quarta M, Mukherjee S, et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020;11(1):1545.
- Ocampo A, Reddy P, Martinez-Redondo P, et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016;167(7):1719-1733.e12.
- Yang J-H, Petty CA, Dixon-McDougall T, et al. Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY). 2023;15(13):5966-5989.
- Lu YR, Tian X, Sinclair DA. The Information Theory of Aging. Nat Aging. 2023;3: 1486-1499.
- Yücel AD, Gladyshev VN. The long and winding road of reprogramming-induced rejuvenation. Nat Commun. 2024;15(1):1941.
- Macip CC, Hasan R, Hoznek V, Kim J, Metzger LE, Sethna S, Davidsohn N. Gene Therapy Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cell Reprogram. 2024;26(1):24-32. doi:10.1101/2023.01.04.522507
- Drake SS, Mohammadnia A, Heale K, et al. Cellular rejuvenation protects neurons from inflammation mediated cell death. bioRxiv. 2023:2023.09.30.560301. doi:10.1101/2023.09.30.560301
- Tai WL, Cho K-S, Kriukov E, et al. Suppressing DNMT3a Alleviates the Intrinsic Epigenetic Barrier for Optic Nerve Regeneration and Restores Vision in Adult Mice. bioRxiv. 2023:2023.11.17.567614. doi:10.1101/2023.11.17.567614
- Tribble JR, Kokkali E, Otmani A, et al. When Is a Control Not a Control? Reactive Microglia Occur Throughout the Control Contralateral Pathway of Retinal Ganglion Cell Projections in Experimental Glaucoma. Transl Vis Sci Technol. 2021;10(1):22.
- Rojas B, Gallego BI, Ramírez AI, et al. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J. Neuroinflammation 2014;11:133.
- Samuel MA, Zhang Y, Meister M, Sanes J. R. Age-Related Alterations in Neurons of the Mouse Retina. J Neurosci. 2011;31(44):16033-16044.
- Ferdous S, Liao KL, Gefke ID, et al. Age-Related Retinal Changes in Wild-Type C57BL/6J Mice Between 2 and 32 Months. Invest Ophth Vis Sci. 2021;62(7):9.
- Chaqour B, Duong TT, Yue J, et al. AAV2 vector optimization for retinal ganglion cell-targeted delivery of therapeutic genes. Gene Ther. 2024;31(3-4):175-186. doi:10.1038/s41434-023-00436-8
- Nieuwenhuis B, Laperrousaz E, Tribble JR, et al. Improving adeno-associated viral (AAV) vector-mediated transgene expression in retinal ganglion cells: comparison of five promoters. Gene Ther. 2023;30(6):503-519.
- Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020;12(573):eaaz7423.
- Pellissier LP, Hoek RM, Vos RM, et al. Specific tools for targeting and expression in Müller glial cells. Mol Ther Methods Clin Dev. 2014;1:14009.
- Xu Q, Rydz C, Huu VAN, et al. Stress induced aging in mouse eye. Aging Cell. 2022;21(12):e13737. doi:10.1111/acel.13737
- Poganik JR, Zhang B, Baht GS, et al. Biological age is increased by stress and restored upon recovery. Cell Metab. 2023;35(5):807-820.e5.
Comments
The comment section on the IGR website is restricted to WGA#One members only. Please log-in through your WGA#One account to continue.Log-in through WGA#One

