Prostaglandin-Associated Periorbitopathy (PAP): Clinical features, pathophysiology and implications for ophthalmologists


by Stanley J. Berke and Joseph Tan
We have performed a comprehensive search using Pubmed to identify all reported cases of Prostaglandin-Associated Periorbitopathy (PAP).1 Ten articles were found in the English literature dating back to 2004, reporting 39 cases of PAP: 23 cases resulting from bimatoprost use, eight cases from travoprostuse, and eight cases from latanoprost use.2-11 These articles were systematically reviewed. Five articles that shed light on the pathophysiology of PAP were identified.13-17 Of the ten articles reviewed, there were a total of 37 patients with PAP: 17 males (44%) and 22 females (56%). The mean age of onset is 69 years (37 to 84 years). Eight cases were reported in a Japanese journal, two cases in a Korean journal, and the rest were in English journals.
Clinical features
The following clinical features of PAP have been described1 (Fig. 1):
- Upper lid ptosis
- Deepening of upper lid sulcus
- Involution of dermatochalasia
- Orbital fat atrophy
- Mild enophthalmos
- Inferior scleral show
- Reduction of the inferior eyelid bags
- Tight orbits
These effects arise after a period of several weeks to several years on treatment with PGAs.
Mechanism of PAP
Most of the anatomical changes of PAP are thought to be due to orbital fat Atrophy,7,14-16 while dehiscence of the levator aponeurosis or Muller's muscle may account for the upper lid ptosis.2
Histopathologic studies of the pre-aponeurotic orbital fat taken from patients who developed deep upper eyelid sulci while on prostaglandin analogue treatment revealed that the mean adipocyte density was higher in treated eyes compared with untreated eyes.7 The orbital fat cells were shrunken and more densely packed in treated eyes. This effect was statistically significant for bimatoprost and travoprost, but not latanoprost. This finding supports that orbital fat atrophy occurs following use of PGAs.
The mechanism of fat atrophy appears to be related to the effect of Prostaglandin F2alpha (PGF2 alpha) on adipocytes. Studies have shown that PGF2 alpha inhibits adipogenesis by activating mitogenactivated protein kinase. This causes inhibitory phosphorylation of a nuclear ormone receptor called peroxisome proliferator-activated receptor gamma (PPAR gamma), which is central to adipocyte differentiation.14,15 Inhibition of adipocyte differentiation and intracytoplasmic lipid accumulation by this mechanism may be responsible for the resultant fat atrophy. Reversal of fat atrophy after PGF2a cessation may be ue to removal of this inhibiting agent, allowing adipogenesis to recommence. In vitro studies comparing the different PGAs showed that bimatoprost had the most anti-adipogenic effect and latanoprost had the least.16
The mechanism for blepharoptosis is less clear. Immunohistochemistry studies have shown a decrease in collagen types I, III, and IV in the ciliary body of monkey eyes treated with PGF2 alpha compared with untreated eyes.17 It is possible that PGAs could also decrease the amount or weaken the structure of collagen in the levator aponeurosis or Muller muscle ligament, resulting in ptosis.
Potency of PAP effect among PGAs
Bimatoprost has the most anti-adipogenic effect among thePGAs, followed by travoprost. Latanoprost has very little effect. This is supported by the observation that most reports of PAP arise from bimatoprost, followed by travoprost and latanoprost. The latency of effect is also shortest in bimatoprost, and longest in latanoprost. Nakakura et al. even reported improvement of bimatoprost and travaprostinduced PAP after switching to latanoprost,8 indicating that the PAP effect of latanoprost is weak.
Pharmacokinetic studies showed that after the topical administration of 0.1% imatoprost on eyes of male cynomolgus monkeys, the drug concentration in eyelid tissue is 2000 times higher than in aqueous, and 16 times higher than in iridociliary tissue.13 This indicates significant periorbital absorption of bimatoprost, which may contribute to its strong tendency to cause PAP.
Latency of onset of PAP
The onset of PAP varies depending on the patient and type of PGA used. The earliest signs of PAP have been seen in patients taking bimatoprost for one month.2 In the study by Park et al., the mean duration of eye-drop usage was 2.4 ± 0.8 years in bimatoprost users, 4.8 ± 2.3 years in travaprost users, and 5.9 ± 3.6 years in latanoprost users.7 It is likely that signs of PAP occur earlier than indicated here, but the effects may not be noticed until much later.
Reversibility of PAP
Many authors have reported reversal of PAP after discontinuation of bimatoprost and travoprost. Reversal of upper eyelid sulcus deepening has been reported to occur anywhere from four weeks to nine months after cessation of bimatoprost therapy.2-4,6,8 The signs of travoprost- induced PAP have been reported to resolve anywhere between two to 15 months after discontinuation of travoprost.5,8 Of the two existing articles reporting latanoprost-induced PAP, the medication was not discontinued, so reversal could not be noted.7,9 It is possible that some cases PAP after long-term use may not be reversible.
Clinical implications of PAP
The anatomic changes to the periorbital soft tissues should not be taken lightly. It can be cosmetically unappealing and especially noticeable in monocularly treated patients. There have been reports of patients who presented for oculoplastics evaluation for correction of the asymmetry. 3,6 PAP has implications beyond just the cosmetic effect. Asymmetry of the orbits and eyelids has led to unnecessary imaging and workup for causes of unilateral enophthalmos or apparent contralateral proptosis.3,6
The deep orbits and tight eyelids present a challenge to eye care clinicians when examining the eye and performing procedures. Goldmann applanation tonometry, for instance, is more difficult to perform as both the patient and the clinician have difficulty lifting up the patient's ptotic upper eyelid. Any procedure performed on the superior globe becomes more challenging, such as laser suturelysis, bleb needling and laser trabeculoplasty. There is evidence suggesting that PAP may lead to refractory glaucoma. Lee et al.12 reported six cases of glaucoma patients with 'tight orbit syndrome' who had elevated IOP refractory to medical and surgical therapy, with advanced visual field loss. One patient in their series had improvement of IOP after orbital decompression. Although an association between the tight orbits and prostaglandin use was not mentioned in this article, all of the patients had been on chronic prostaglandin therapy.
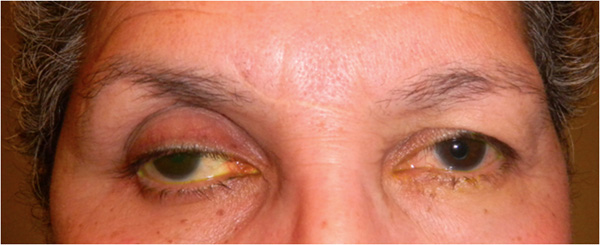
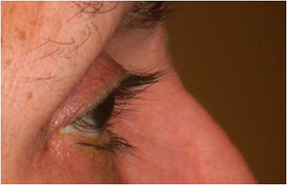
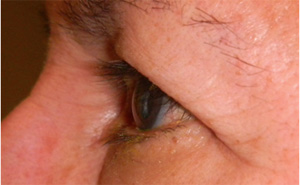
Fig. 1. A 67-year-old Hispanic female with history of right eye angle recession glaucoma treated with travoprost for 15 years. Top and bottom left: Note deepening of the upper lid sulcus, involution of dermatochalasis, ptosis, inferior scleral show, and flattening of the inferior lid bulge on the right compared with the left side. Bottom right: Left eye in comparison.
References
- Pasquale LR, Berke SJ. Prostaglandin-Associated Periorbitopathy. Glaucoma Today 2011; 9(3): 51-54.
- Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci 2004; 81(8): 574-577.
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg 2008; 24(4): 302-307.
- Yam JC, Yuen NS, Chan CW. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol Ther 2009; 25(5): 471-472.
- Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol 2009; 53: 176-179.
- Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy - an unfamiliar side effect of prostaglandin analogues. Orbit 2010: 29; 357-359.
- Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol 2011; 55(1): 22-27.
- Nakakura S, Tabuchi H, Kiuchi Y. Latanoprost therapy after sunken eyes caused by travoprost or bimatoprost. Optom Vis Sci 2011; 88(9): 1140-1144.
- Ung T, Currie ZI. Periocular changes following long-term administration of latanoprost 0.005%. Ophthal Plast Reconstr Surg 2012; 28(2): e42-e44.
- Noma K, Kakizaki H. Bilateral upper eyelid retraction caused by topical bimatoprost therapy. Ophthal Plast Reconstr Surg 2012; 28(2): e33-e35.
- Sira M, Verity DH, Malhotra R. Topical bimatoprost 0.03% and iatrogenic eyelid and orbital lipodystrophy. Aesthet Surg J 2012; 32(7): 822-824.
- Lee GA, Ritch R, Liang SY, et al. Tight orbit syndrome: a previously unrecognized cause of open-angle glaucoma. Acta Ophthalmol 2010: 88(1): 120-124.
- Woodward DF, Krauss AH, Chen J, et al. Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). J Pharmacol Exp Ther 2003; 305: 772-785.
- Lepak NM, Serrero G. Prostaglandin F2 alpha stimulates transforming growth factor-alpha expression in adipocyte precursors. Endocrinology 1995; 136: 3222-3229.
- Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem 1998; 23; 273: 1855-1858.
- Choi HY, Lee JE, Lee JW, Park HJ, Lee JE, Jung JH. In Vitro Study of Antiadipogenic Profile of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in Human Orbital Preadipocytes. J Ocul Pharmacol Ther 2012; 28(2): 146-152.
- Sagara T, Gaton, DD, Lindsey JD, et al. Topical prostaglandin F2α treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol 1999; 117: 794-801.

