advertisement


Direct Visualization of RGC Apoptosis In Vivo
M. Francesca Cordeiro
Apoptosis is an orchestrated form of cell death in which cells commit suicide. Its occurrence has been linked to the pathogenesis of disease mechanisms throughout the body. This is exemplified in the condition of glaucoma where retinal ganglion cell (RGC) apoptosis is strongly implicated in the development of vision loss. However, RGC degeneration occurs well before current visual function tests detect abnormalities and until now, RGC apoptosis has only been demonstrated histologically.
We have devised a new, non-invasive real-time imaging technique to visualize individual RGC apoptosing in vivo. We have demonstrated this dynamic process using confocal laser scanning ophthalmoscopy and fluorescent-labeled annexin 5. We have been able for the first time to image changes occurring in RGC apoptosis over hours, days and months in vivo, using different experimental glaucoma-related models, including optic nerve transection, chronic ocular hypertension and drug-induced RGC apoptosis. We have shown effects depend on the magnitude of the initial insult.
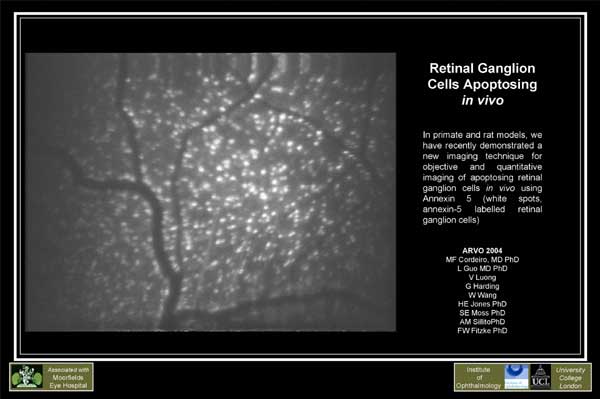
This novel technique is an important advance in our ability to monitor
the disease mechanism in experimental glaucoma and ocular disorders. It
has potential as a new diagnostic and screening test with which to identify
patients with early glaucoma, before they lose vision. It also opens the
door to directly observing effects of neuroprotective therapeutic strategies
in glaucoma. Imaging RGC apoptosis in vivo will potentially revolutionize
our treatment and management of blinding and nervous diseases.
Presented at ARVO 2004 by M.F. Cordeiro, L. Guo, V. Luong, G. Harding, W. Wang, H.E. Jones, S.E. Moss, A.M. Sillito, F.W. Fitzke of the Institute of Ophthalmology, London, UK

Comment on Cordeiro et al., Direct Visualization of RGC Apoptosis In Vivo
Martin B. Wax
Our knowledge of glaucoma has evolved such, that we obtain a better understanding of the disease with each new tool we use to study its pathogenesis. Our present understanding reveals a characteristic time course in which achromatic visual field deterioration is proceded by optic nerve atrophy (i.e., cupping), which reflects accumulated atrophy of the nerve fibers layer of the inner retina. With such a schema, it is reasonable to assume that defects in retinal cellular metabolism, in response to glaucomatous stress conditions, are present, and amenable to detection even prior to NFL fiber drop out. Furthermore, such abnormal cellular metabolism might serve as predictor of RGC cell death.
Although there are several technologies that aspire to being able to predict RGC damage or death, the work of Cordeiro et al. appears to have made major inroads in characterizing what might be considered the next important signaling hurdle, namely, the direct detection of RGCs undergoing apoptosis. In this case, detection is based on real-time annexin labeling and is performed non-invasively. Annexin, a phospholipid binding protein, externalizes on the cell surface only if the cellular apoptosis process is active. Thus, annexin labeling is thought not possible before the programmed cell death cascade is triggered by the activation of caspases, or after the cell involutes into an apoptotic body waiting to be swept away by the immune response that restores tissue homeostasis.
Have the authors indeed performed the first ocular studies with fluoresceinated
annexin that they say "will potentially revolutionize our treatment and
management of blinding and nervous diseases"? Using scanning confocal ophthalmoscopy
to identify fluoresceinated annexin (FA) binding of RGCa undergoing apoptosis
cell death after intravitreal delivery of FA, the authors have used three
models to elicit RGC death. These models included a chronic OHT rat model,
a staurosporine-induced toxic retinopathy model in rats and monkeys, and
an optic nerve transaction model in rats. In these cases, a robust signal
was observed that seems highly amenable to objective quantitation and analysis.
However, in order to become the highly desireable and useful tool that the
authors envision, several obstacles must be overcome. First,
the fluoresceinated annexin must be delivered by a more user friendly method
(i.e., oral or intravenous) and not by the intravitreal route that the authors
used to show proof of concept. This will require considerable scale-up manufacturing
efforts as well as having the agent clear all the hurdles in order to be
approved for human use. Second, in order to be highly useful, the
technique should ideally be able to detect a rather small number of ganglion
cells dying at any one time in the glaucomatous process, and render this
amount convincingly distinguishable from the constituitive level of RGC
cell death that accompanies the aging process. Thus, the signal to noise
ratio of the apoptotic signal may well depend on the activity of the disease
process. The signal may well be below the threshold for meaningful discrimination
many times during the typical two decade-long clinical course exhibited
by many patients with glaucoma.
As a counterpoint, one might advocate that the presence of some early glaucoma defects such as nasal steps or Seidel scotomas could be the result of clusters of RGCs that undergo apoptosis at about the same time. This might be fortuitous for a fluorescent detection device as clustered RGC loss might provide a good signal for monitoring. Therefore, it is not so far fetched to think that we might have, in relatively short order, an office-based device (not unlike one that performs fluorescein angiography) to use for managing glaucoma patients that relies upon real-time detection of RGCs undergoing apoptosis. Such a device will, of course, have to be validated within the context of our other current technologies in order for us to learn how to best translate the new information it provides, into our decisions that seek to positively effect patient outcome.
Lastly, in addition to hopefully serving as a powerful new clinical tool, the authors provide a glimpse of a device that holds great research promise by allowing for the study of potential neuroprotective drugs using meaningful endpoints that are based on the direct assessment of RGC death. Such a device might potentially serve as a surrogate biomarker that can predate and predict the outcomes obtained by visual field studies or other key endpoints currently utilized by the FDA. Thus, clinical outcome studies might be shorter in duration than those that rely solely on visual field outcomes. The utilization of such a diagnostic device in our clinical practice or as a research tool to design drugs that thwart somatic- or axonal-initiated RGC death, would indeed go a long way in justifying the 'revolutionary' status that the authors confidently hope the technology will earn in the near future. In the meantime, it is easy to see why their excitement is warranted.
In the Editor's Selection of IGR 4-3 the work of Naskar et al. was discussed. They monitored retrogradely labeled ganglioncells using fluorescence imaging microscopy. This technique requires injection of dye into the superior colliculus which is not applicable in humans. Since most of the tracer dyes used for retrograde abeling are highly lipophilic and can traverse cell membranes, some investigators feel that they are not the best choice to accurately detect solely RGCs in the rodent ganglion cell layer which also contains numerous displaced amacrine cells. Furthermore, some of these dyes have been shown to induce apoptosis directly. These are very important concerns when evaluating the results of quantitative studies of RGC loss in experimental glaucoma if retrograde labeling is used.
