advertisement

Martin B. WaxGlaucoma therapy is unique in that the sites for drug delivery span both the anterior and posterior segments. The anterior segment is not only the target of palliative therapy for drugs that lower IOP, but is also the site of the disease pathogenesis itself; namely, the lesion(s) in the outflow pathways that result in impaired aqueous outflow in most forms of open angle glaucoma. The posterior segment is equally important as a therapeutic site as we look towards the future. There are many reasons to anticipate that we will inevitably be able to deliver drugs to the retina and optic nerve that protect, facilitate repair, or regenerate diseased neuronal or glial tissues that contribute to glaucomatous optic neuropathy and act by mechanisms that are independent of the retinal stress that occurs in the presence of elevated IOP. The doorway to future therapeutics that targets other retina stressors thought to be relevant to glaucoma such as ischemia, excitotoxity, and aberrant immunity has now been opened. Each symposium speaker shared their thoughts regarding important issues of drug delivery that need to be considered as we further open the door to improved therapeutics for the treatment of glaucoma. Harry Quigley (Johns Hopkins) focused his talk on the main limitations of topical drops, which is the issue of compliance and adherence. Of the possible drug delivery approaches that can be taken (administered by the patient or administered by the doctor or office staff or surgically placed) it is certainly topical drops that is not only the most popular for glaucoma, but unfortunately the one that provides the least assurance that a patient will receive a desired medication as the prescribing physician intended. Hank Edelhauser (Emory) reminded us that the cornea comprises the greatest barrier to entry for topical drops. The cornea is a layered structure consisting of an epithelial layer on the outer front side, the stroma in the center, and a thin endothelial layer on the inner surface. There are also two membranes (Bowman's and Decemet's) that separate these three layers. The outer epithelial layer (five layers of cells) is lipophilic, whereas the stroma, which accounts for about 90% of the thickness, is hydrophilic (it contains almost 80% water). The endothelial layer (one cell thick) is slightly lipophilic. In addition, the mucin that coats the cornea serves to prevent adhesion of proteins, bacteria and other foreign substances. So, in order for a drug to penetrate the cornea, it must have a balance of physicochemical properties that enable it to successfully cross each of these layers:
Hank also reminded us that the human sclera is more permeable than the cornea to many hydrophilic and hydrophobic drugs. Furthermore, the rate of diffusion is determined by molecular mass and size. Intrascleral injection does not seem practical as drug delivery method because of small volume that is possible with such an approach. While drug diffusion through the cornea is not very efficient, the rate of drug diffusion through the sclera is significantly higher, roughly equal to the cornea denuded of corneal epithelium. Further, the surface area of the sclera (approximately 17 cm2) is a lot bigger than the cornea (approximately 1 cm2). Thus, an effective case was made that perhaps the most compelling location from which to deliver sustained drugs to the eye (either anterior or posterior) may be the scleral surface. Alan Weiner (Alcon) provided an overview of various properties that should be associated with drug delivery devices (i.e., DDD) in order to be successful in treating glaucoma through lowering of intraocular pressure (i.e., IOP). For patient 'self administration' systems, the DDD should provide drug therapy for one week or longer. Such DDDs should have minimal side effects, should not impair vision, must stay in place, and be easy to administer and remove by the patient. For 'in-office' systems, the DDDs should provide drug therapy for three to six months, be a reimbursable procedure, have minimal side effects, not impair vision, must stay in place, and be easy to administer and remove by the physician. For surgical implantation systems, the DDDs should provide drug therapy for more than one year, be a reimbursable procedure, have minimal side effects, not impair vision, must stay in place, and be easy to administer and remove (should the need arise) by the physician. Alan indicated that materials being considered for short-term delivery of drugs (i.e., hours to days) included various gels and liquid biopolymers, while for long-term delivery of drugs (days to weeks and beyond) semisolid polymers and microspheres have been under evaluation. One of the problems to be overcome by different DDDs is the need to provide sufficient bioadhesion to stay in place, while retaining properties that would allow for its removal. Alan also discussed the numerous potential sites (fig. 1) that are possible for commercial products that can be designed to deliver specific payloads of drug into the eye that range from punctual plugs to subretinal implants. The possible sites of drug delivery that may targeted for glaucoma therapeutics are shown below and highlighted in red. Fig 1. Potential sites for drug delivery (in red). (Courtesy Alan
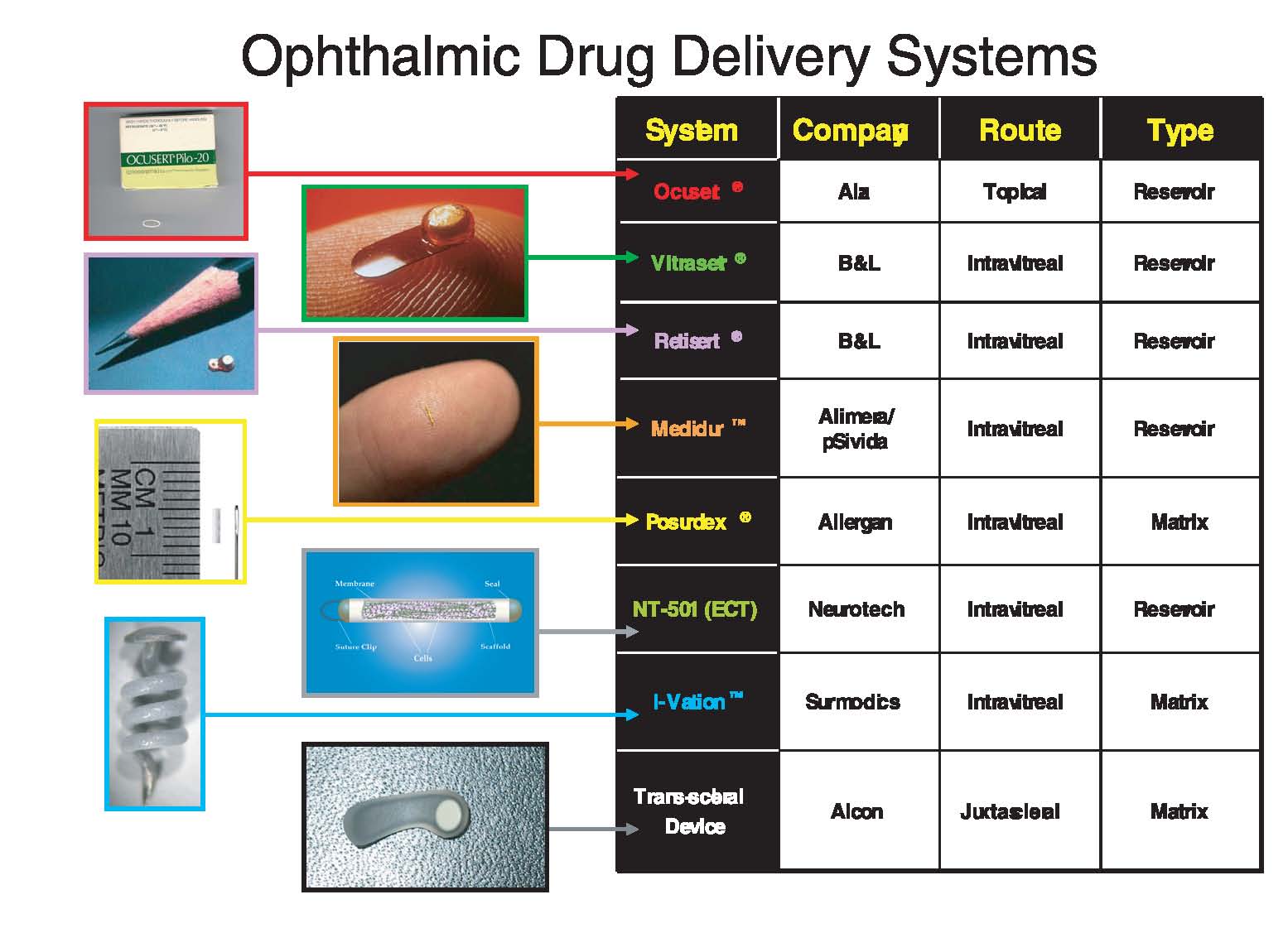
Weiner.) Alan further provided an overview of several devices (fig. 2, p. 170) that are currently available or in clinical studies for several ophthalmic indications in an effort to stimulate the thinking of the glaucoma community as to which of these approaches, if any, might be useful for treating glaucoma. Examples of devices and their target indications that were discussed are shown below. Fig. 2. Overview of drug delivery devices. (Courtesy Alan Weiner.)
Vince Lee (USC) spoke on the use of self-assembling peptides that form nanofibers with nanopores as controlled drug delivery devices for small molecules, proteins, RNA and DNA. Amphiphilic peptides form self-assembling nanofibers with a hydrophobic side and a hydrophilic side. The hydrophobic side of the nanofibers forms a double sheet within the fiber, while the hydrophilic side form the outside of the nanofibers that can interact with water molecules to form a hydrogel with a water content as high as 99.9%. The amphiphilic peptides can be programmed to undergo self-assembly in aqueous solutions to form nanofibers that associate into nanofiber scaffolds. These nanofiber scaffolds contain 5-200 nm pores and have a very high water content (i.e., > 99.5%). They mimic 3-D extracellular matrices, and thus can be used as 3-D cell cultures for tissue engineering and as drug delivery devices which allow for the slow diffusion of drugs to sites of interest. Nanoparticles can be used as drug delivery devices for the front of the eye because they form transparent gel-like structures that don't scatter light. Vince concluded by discussing the potential to deliver drug 'on-demand' with Wi-Fi controlled implantable pumps. Steve Dowdy (UCSD) discussed the use of small interfering RNA (siRNA) as a burgeoning drug delivery technology. Small interfering RNA's are one of the most recent additions to the wide repertoire of nucleic acid molecules used to silence gene expression. The robustness of this approach has prompted numerous biotechnology and academic institutions to develop siRNA libraries for genome-wide screening in mammalian cells. However, the potential of siRNAs to be used as potential therapeutic agents is unfortunately limited by one key intrinsic property of siRNAs which is their residual negative charge. As a result, they do not willingly enter cell membranes which are also negatively charged on their surface. Their ability to incorporate into cells and internalize to the cytoplasmic domain is a prerequisite to their gene silencing abilities. Steve presented several theoretical approaches that many laboratories are exploring in order to neutralize the net negative overall charge of siRNA molecules, thus rendering them 'friendlier' to cell membranes and thus permit them to internalize by macropinocytosis in order to accomplish their therapeutic objective. Finally, Weng Tao (Neurotech) presented a novel method of delivering peptide-based therapeutics to target cells. One limitation to the delivery of biologicals such as proteins and peptides is that unlike conventional small molecules, biologicals are highly susceptible to degradation by proteases and other enzymes that reside in living tissues. Neurotech's Encapsulated Cell Technology (ECT) is a drug-delivery platform that allows the delivery of virtually any secreted protein, including potent neurotrophic factors, to the retina and other ocular tissues. This drug-delivery platform is broadly applicable to major forms of ocular disease, including: retinal degeneration, angiogenesis, and inflammation as well as neuroprotection as might be desirable in glaucoma. The ECT is an implantable device containing cells encapsulated within a semi-permeable membrane, thus isolating them from the vitreous and minimizing immune rejection, but allowing therapeutic agents produced by the cells to diffuse through the membrane. Cells can be bio-engineered to allow controlled, continuous, long-term delivery of secreted neurotrophic factors or cytokines, allowing this drug-delivery platform to have utility in treating various retinal diseases as well as other ocular indications. The device is implanted in the eye under local anesthesia during a 15-minute procedure. Implants can be retrieved, providing an added level of safety as well as the ability to reverse or adjust therapy, if needed. The feasibility of such an approach is not just theoretical. Neurotech has completing Phase 1 clinical trials with ciliary neurotrophic factor (CNTF) in retinitis pigmentosa patients and is currently in advanced clinical trials for RP and AMD. It is worth noting here that this technology is theoretically applicable to any secreted protein. Some molecules that immediately come to mind are BDNF for ganglion cell protection and rod-derived cone survival factor for photoreceptor protection in AMD or RP. Conventional delivery of such molecules requires creating an appropriate cell line to secrete the product, followed by purification and validation in large quantities for packaging and delivery. With the Neurotech device, once the cell line is created and validated by QA for secretion and duration of viability, no matter which protein, the cells are placed in the device. The biologicals are manufactured in situ at a nanoscale and are delivered to the target tissue fresh off the ribosome with no impurities or degradants or chemical muss and fuss. The ease and general applicability inherent in this therapeutic approach have enormous potential payoff with respect to both efficiency and long term cost if indeed the engineered cell lines can deliver payload effectively for prolonged periods of time. Conclusion
The future for glaucoma therapeutics is very bright indeed.
Opportunities are being developed that aim to deliver drugs in a
sustained manner for prolonged periods of time that rely less on
individual patient administration and therefore obviate the compliance
and adherence issues that are the bane of topical drop administration.
It is anticipated that some of the delivery options presented in this
excellent symposium will soon be realized for therapies that are both
palliative as well preventative. Ideally, the goal of all drug delivery
approaches is to target glaucomatous pathogenic mechanisms directly and
thus prevent the onset of disease in the trabecular meshwork of the
anterior segment and/or the retinal and optic nerve tissues of the
posterior segment.
|