advertisement

Scientific photos
En face view of 3D segmentation of the lamina cribrosa from a normal human donor right eye
J. Crawford Downs, Christopher A. Girkin, and Claude F. Burgoyne
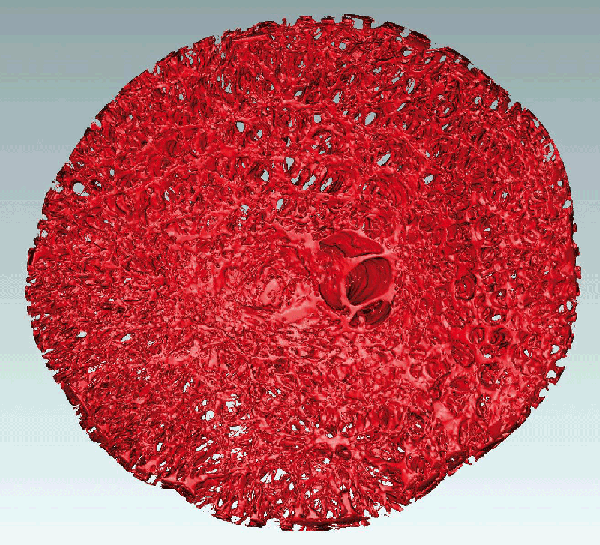
The 3D ONH reconstruction was generated using a custom-built serial section-based episcopic fluorescent image capture device in Dr. Downs' laboratory wherein the trephinated ONH and peripapillary sclera is embedded in light-blocking paraffin, then serial sectioned at 1.5 micrometer resolution on a microtome. After each section is taken, the tissues exposed on the paraffin block face are imaged using an attached fluorescent microscope, which captures the inherent cellular and connective tissue autofluorescence at 1.5x1.5 micrometer pixel resolution using a 16-megapixel camera covering a 6.2x6.2 mm field of view. The serial section images are automatically registered and stacked, and the resulting 3D ONH reconstructions (up to 45 GB of data per eye at 1.5x1.5x1.5 micrometer voxel resolution) encompass all the tissues from the internal limiting membrane through the retrolaminar optic nerve. Dr. Girkin's laboratory delineates each 3D ONH reconstruction into its constituent tissues (lamina, sclera, prelaminar neural tissues, etc.) using custom software developed in Dr. Burgoyne's laboratory. The lamina cribrosa connective tissues seen in the photo were then segmented from the surrounding neural tissues using a previously published method (Grau, et al. IEEE Trans Med Imag, 25(3):245-255, 2006).
These three-dimensional ONH reconstructions are being used to determine the differences in ONH and laminar structure between young and old human donors of European descent, as well as between donors of African and European descent. These structural differences, as well as those predicted by eye-specific biomechanical models based on each 3D reconstruction, will help explain the significantly increased risk of glaucoma in the elderly and black populations.
Acknowledgements: NIH Grants R01 EY018926 (JCD/CAG); R01 EY019333 (JCD/CAG); R21 EY018152 (JCD).
J. Crawford Downs,PhD
Ocular Biomechanics Laboratory
Devers Eye
Institute
1225 NE 2nd Avenue
Portland, OR 97232
USA
Claude Burgoyne, MD
Optic Nerve Head Research Laboratory
Devers
Eye Institute
1225 NE 2nd Avenue
Portland, OR 97232
USA
Christopher Girkin, MD
Department of Ophthalmology
School of Medicine
University of Alabama at Birmingham
700 S. 18th Street, Suite
601
Birmingham, AL 35294-0009
USA
The superior retina of a CX3CR1 GFP/+ transgenic mice imaged in vivo with a confocal scanning laser ophthalmoscope (CSLO)
Christopher Leung
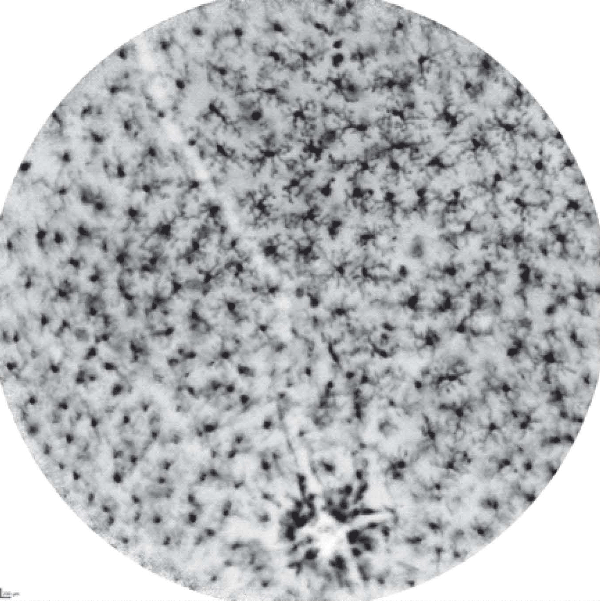
CX3CR1 is expressed by the retinal microglia and functions as a chemotactic receptor for fractalkine, which has been implicated in the communication between neurons and microglia and the control of microglial neurotoxicity. By replacing one copy of the fractalkine receptor gene with a copy of the green fluorescent protein (GFP) gene, it is possible to detect the fluorescent microglia in vivo. In the resting state, retinal microglia exhibit a ramified form with cellular processes extending from the cell bodies.
Dr. Christopher Leung
Department of Ophthalmology and Visual Sciences
The Chinese University of Hong Kong
3/F, Hong Kong Eye Hospital
147K
Argyle Street
Hong Kong
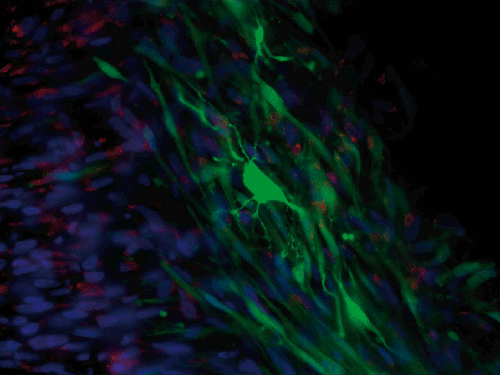
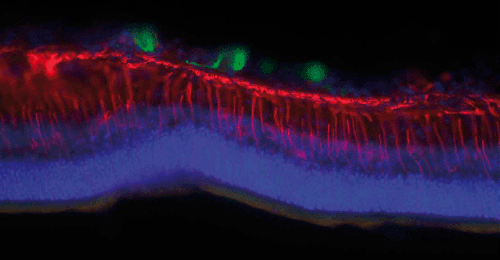
Stem cells
Keith Martin and Natalie Bull
Stem cells (green) derived from human retinal M'ller (glial) cells transplanted into the vitreous of a rat eye with glaucoma. One of the stem cells has differentiated/become a neuronal-like cell and extended branches of fine processes to connect to other cells, essentially making a new neuron after transplantation. Cell nuclei are counterstained in blue, and we can see other retinal cells to the left of the green graft, and red shows a glial marker stain.
Stem cells (green) derived from human retinal M'ller (glial) cells transplanted into the vitreous of a rat eye with glaucoma. The cells settle on the inner surface of the retina and sometimes extend processes, but are prevented from migrating into the neural retina by a barrier primarily formed by the retinal M'ller glia (stained for nestin expression in red). In order to replace retinal ganglion cells (neurons) lost in glaucoma we need to find a way to modify this glial barrier in order for our transplanted cells to migrate into the tissue (counter-stained with a nuclear marker in blue) where they are needed to form new connections.
Prof. Keith Martin and Dr. Natalie Bull
Centre for Brain Repair
University of Cambridge
Forvie SiteRobinson Way
Cambridge CB2 0PY
UK
Subconjunctival tissues
Tina Wong
Untreated
MMC Treated
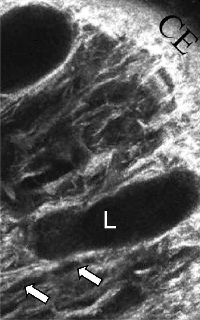
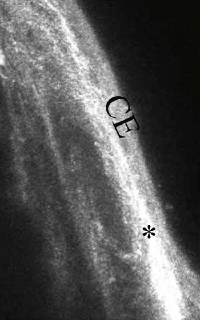
Demonstration of the appearance of subconjunctival tissues in a functioning, filtering bleb by confocal microscopy imaging in a mouse model of glaucoma filtration surgery. At day 28 post-surgery, the conjunctiva at the surgical site of the untreated control eye appeared scar-like with deposition of a dense white connective tissue with no clear spaces (*). The MMC-treated animal clearly shows multiple lacunae (L) and cysts (white arrows) within the subconjunctival space thus demonstrating the site of filtration in the bleb. These observed lacunae and conjunctival cysts within the bleb has been also been previously shown in MMC-treated human filtering blebs by confocal imaging. The very similar appearances observed in the mouse and human eyes make this surgical model of glaucoma filtration surgery an invaluable tool when evaluating the effect of new antifibrotic target molecules on post-operative subconjunctival scarring. CE, conjunctival epithelium; S, sclera.(Seet et al. Validation of the glaucoma filtration surgical mouse model for anti-fibrotic drug evaluation. Journal of Molecular Medicine 2011, E Pub Jan 11).
Dr. Tina Wong
Singapore National Eye Centre
Singapore Eye Research
Institute
11 Third Hospital Avenue
Singapore 168751

