advertisement

Scientific photos
Reversal of lamina cribrosa displacement after trabeculectomy
Tae-Woo Kim, Eun Ji Lee and Robert N. Weinreb
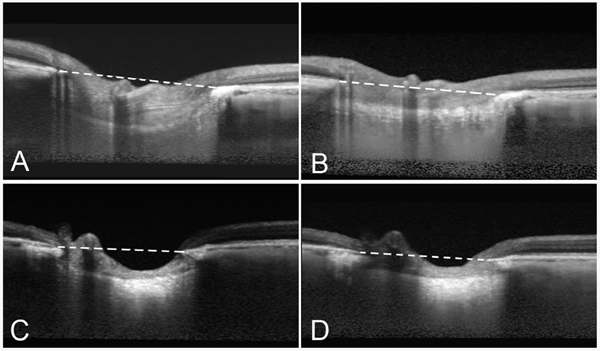
Preoperative and postoperative optic nerve images of the left eye of a 18-year old (A, B) and an 81-year old woman (C, D), where the IOPs were lowered from 25 to 6 mmHg and from 20 to 7 mmHg, respectively. The reversal of the backward bowing of the lamina cribrosa is clearly noticeable. Images were taken by enhanced depth imaging SD-OCT. Dashed lines indicate the plane of Bruch's membrane opening.
Tae-Woo Kim
Department of Ophthalmology
Seoul National
University Bundang Hospital
Seoul National University College
of Medicine
166 Gumi-dong
Bundang-gu, Seongnam, Gyeonggi-do
463-707
Korea
Shiley Eye Center
University of California, San Diego
9500 Gilman Drive 0946
La Jolla, CA 92093-0946
USA
Quantum dots trace lymphatic drainage from the mouse eye
Neeru Gupta
Most glaucoma therapies attempt to improve drainage through aqueous outflow pathways. Lymphatic channels drain extracellular fluid, solutes and proteins in most organs. We have reported the presence of lymphatics in the human eye and sheep eye using cell-specific markers.1 More recently, we developed a sheep model in which lymphatic drainage from the eye was measured.2 Lymphatics likely contribute to fluid drainage from the eye, and developing methods to visualize lymphatic flow in vivo is relevant to glaucoma studies.
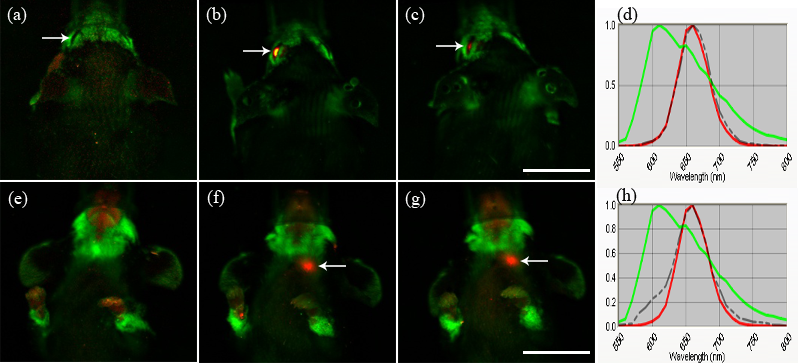
Fig. 1. In-vivo hyperspectral fluorescent imaging of head and neck in dorsal (a-c) and ventral (e-g) views. Imaging performed prior to quantum dot injection (a) shows no signal in the left eye (arrow). At 6 h (b) and 24 h (c) following injection, quantum dot signal in red is noted in the left eye (arrow). Strong quantum dot signal is noted in the left neck region (arrow) at 6 h (f) and 24 h (g), and is not seen prior to injection (e). Scale = 10 mm. Emission spectral profiles of the signals in the eye (arrow in (b)) and in the left neck region (arrow in (f)) at 6 h are shown in dotted lines in (d) and (h), respectively. Spectral profiles of both signals confirm the quantum dot spectral profile (red), compared to the background signal profile (green) (d, h). (Reprinted with permission from Tam AL, Gupta N, Zhang Z, Yücel YH. Nanotechnology 2011; 22: 425101)
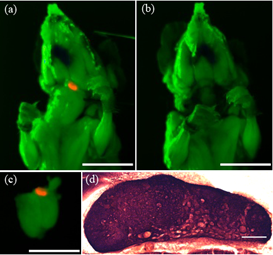
The unique physical characteristics of quantum dots (QDs) are advantageous for long-term single or simultaneous multiple color imaging of live cells and make them suitable for non-invasive in-vivo imaging. We injected QDs into the mouse eye (n = 17) and captured in-vivo images using whole body hyperspectral fluorescence imaging prior to injection, and 5, 20, 40, and 70 minutes, and 2, 6 and 24 hours after injection.3 The use of quantum dots to image this mouse lymphatic pathway in vivo is a novel tool to study potential new treatments to reduce eye pressure and prevent blindness from glaucoma.
- Yücel YH, Johnston MG, Ly T, Patel M, Drake B, Gumus E, Fraenkl SA, Moore S, Tobbia D, Armstrong D, Horvath E, Gupta N. Identification of lymphatics in the ciliary body of the human eye: a novel 'uveolymphatic' outflow pathway. Exp Eye Res 2009; 89: 810-819.
- Kim M, Johnston MG, Gupta N, Moore S, Yücel YH. A model to measure lymphatic drainage from the eye. Exp Eye Res 2011; 93: 586-589.
- Tam AL, Gupta N, Zhang Z, Yücel YH. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology 2011; 22: 425101.
Neeru Gupta,
Professor and Dorothy Pitts Chair, Ophthalmology & Vision
Sciences
Laboratory Medicine & Pathobiology, University of Toronto
Director, Glaucoma & Nerve Protection Unit
The Keenan Research Centre,
Li Ka Shing Knowledge Institute
St. Michael's Hospital
Canada
Human primary trabecular meshwork cells
Núria Comes and Terete Borrás
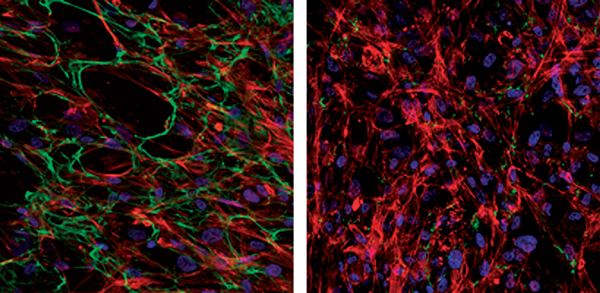
Confocal microscopic images of immuno-fluorescence stained primary human trabecular meshwork cells showing the effects of the angiopoietin- like7 (ANGPTL7) gene on fibronectin and actin networks. Cells from the left panel are non-transfected controls, while those from the right panel are overexpressing the ANGPTL7 gene. Fibronectin (green) and actin (red) were labeled using a mouse anti-human fibronectin antibody under non-permeabilized conditions followed by rhodamine-conjugated phalloidin under permeabilized conditions. Nuclei were stained blue using DAPI. The glaucoma-expressed ANGPTL7 gene prevents fibronectin fibril's assembly and reduces formation of actin stress fibers.
Professor Terete Borrás and Núria Comes
University of Nrth Carolina
at Chapel
Hill Department of Ophthalmology
Chapel Hill, NC 27517
USA

