advertisement

Industry news
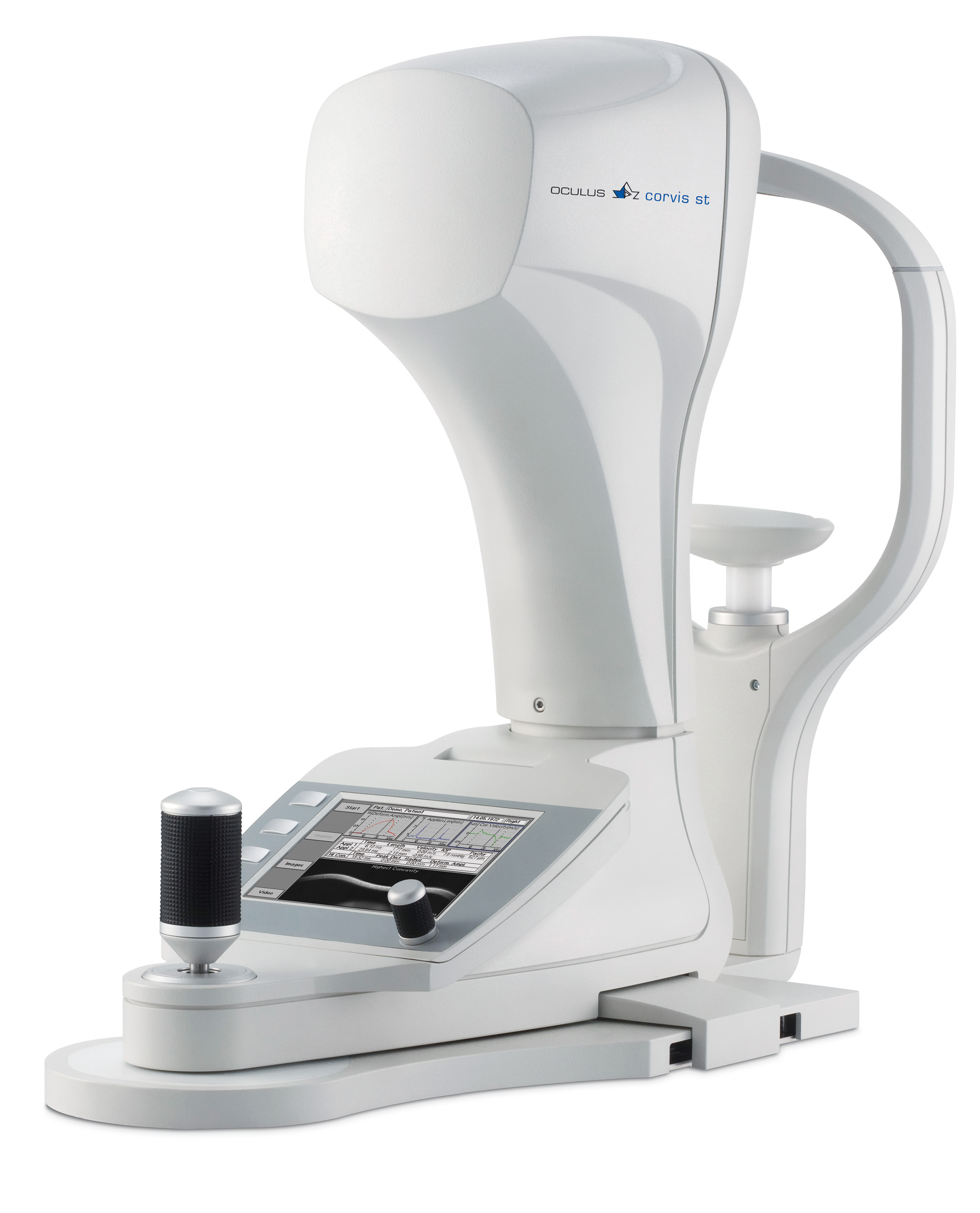
Exciting new non-contact tonometer with high-speed camera - The New Corvis® ST

The OCULUS Corvis® ST is a new non-contact tonometer in combination with an ultra-high-speed Scheimpflug camera (more than 4000 images/ sec) that can visualize the reaction of the cornea on an air impulse.
Conclusions can be drawn regarding the bio-mechanical properties from the response of the cornea after the air impulse. It is well known that IOP-measurements by Goldmann Applanation Tonometry (GAT) and Non-Contact Tonometry (NCT) are influenced by the corneal thickness of the cornea. It is not very well known that the IOP-measurements are even more effected by the biomechanical properties of the cornea. The results of a theoretical study suggest that the potential error of the IOP-readings can be immense: the theoretically predicted values between a stiff and a weak cornea can differ up to 10 mmHg1. Based on the measured IOP, the biomechanical properties and the corneal thickness, the Corvis® ST has the potential to determine a highly accurate value close to the 'true IOP'.
The Corvis® ST can also be used to monitor changes of the deformation response of the cornea caused by, e.g., corneal ectasia and corneal crosslinking. In ectatic corneas the viscoelastic properties of the cornea differs from those in normal corneas. Therefore the parameters that describe the deformation response of the cornea can be used to distinguish between normal and ectatic corneas.
Biomechanical properties of the cornea are also changed due to corneal crosslinking. Although corneal crosslinking is established in clinical practice for a couple of years, it is very difficult to determine if the cornea has become stiffer due to crosslinking. The Corvis® ST enables the user to see significant changes in the deformation response of the cornea after crosslinking. Therefore, the Corvis ® ST will be able to quantify the effect of corneal crosslinking.
The OCULUS Corvis® will be for sale from spring 2012. The device is not for sale in the USA yet, FDA 510K is pending.