advertisement


Management of Glaucoma based on rate of progression and quality of life; lessons from the RCTs

Erik L. Greve and Roger A. Hitchings
OVERVIEW
- IOP reduction works (OHTS, EMGT)
- Lower IOP means better protection against visual loss (EMGT)
- The vast majority of OHT patients does not progress (five years, OHTS)
- Progression may be slow and never affect QoV (EMGT, CNTGS)
- No treatment is without side-effects
- In OHT, the vast majority of patients should only be treated if RoP threatens QoV
- Careful follow-up of untreated patients is imperative
- The most QoV-threatening fact is the presence of advanced VFD
- Progression can be reduced to almost zero by vigorous treatment (AGIS, CIGTS)
- Advanced VFD needs vigorous treatment including, if necessary, cataract extraction
- 20-25% reduction of IOP may not be enough (EMGT)
- Mean age of detected glaucoma patients is just under 70 years. Life expectancy some ten to 15 years
Individualized treatment strategy is based on the fact that:
- there is a large inter-individual variation of RoP
- mean RoP is slow; treatment may not always be of benefit
- Hippocrates: do no harm
��//��
The Glaucoma RCTs (GRCT) have radically changed the perception of glaucoma
as an untreatable disease. Before the GRCT data, there was no high-level
evidence available that glaucoma treatment worked.1,2
Since the GRCTs, it can definitely be concluded that:
-
1. OP reduction is of benefit in OHT/POAG; and
-
2. Lower IOP means better protection against visual field progression.
Questions: -
3.Will treatment inevitably be of benefit?
-
4.Is greater IOP reduction inevitably better?
What is benefit, what is better?
Benefit means avoidance of a decrease in Quality of Vision (QoV), and Quality of Life (QoL).
Risk or Fact
There may either be a perceived RISK for decreased QoV based on statistical data, or measured FACT indicating that Rate of Progression (RoP) will affect QoV.
RISK is based on group data that offer a probability of loss of Quality of Vision that may or may not be applicable to the situation of the individual patient. Therefore, treatment based on FACT is preferable to treatment based on RISK. High IOP in OHT is RISK. We assume that the probability of conversion and subsequent fast RoP is high. FACT exists when the RoP is such that it will reach a level of damage that affects the patient's QoV within a foreseeable time and, of course, within the expected lifespan of the patient. The presence of advanced VFD is still RISK, but a RISK situation where establishing FACT is irresponsible since the next progression step may affect QoV. Mean RoP may be a threat to QoV, even in relatively small defects where the defect is paracentral.
OHT Individualized Treatment
Over 90% of untreated patients did not convert in the OHTS.3 At ARVO 2002, it was reported that:
-
20% of OHTs did have defects on SWAP; some converters may already have had damage, reducing the true number of OHT converters.
-
Conversion is not similar to reduced QoV.
-
Conversion based on optic disc/RNFL changes (OHTS) may come before white/white VF changes. Time from RNFL defect to white/white defect may average five years4; time from HRT change to white/white defect may be 2+ years.5 Even conversion to white/white defect may not have any effect on QoV.
-
A minority of OHT needs treatment for the high RISK of progression to decreased QoV.
-
That minority includes high IOP (mean 30 mmHg, peak 35 mmHg).
-
The vast majority of OHT patients can be treated on the basis of FACT; this means evidence that RoP will affect QoV during the patient's expected lifetime.

Consequences for management (Chart 1):
follow OHT patients carefully with repeated ONH/RNFL and VF measurements. When glaucoma damage appears, continue follow-up and establish RoP. When RoP threatens lifetime QoV, start treatment. Establish RoP on treatment, etc. Establishing RoP requires regular measurements of disc/RNFL or VF: up to three to four times per year.
This approach avoids unnecessary treatment with all its possible effects on QoL. Only those who really need it, because of the threat of QoV, will be treated.
It should be realized that withholding treatment while establishing RoP is only acceptable when the follow-up system is adequate for detecting changes within the time period. Early estimation of RoP will require a more frequent and different follow-up scheme post-diagnosis than usually practiced today.
What is Threat to Quality of Vision?
Decreased QoV is present when progression of defects involving one of the four paracentral locations on threshold perimetry is noted by the patient, or when progression in one eye unmasks a defect in binocular vision.
Assuming progression fits a linear model, knowing the RoP allows the prediction of future field loss and when this will affect QoV.
Threat to QoV is usually based on monocular vision; although the binocular QoV may be good, the chance that other eye diseases may affect QoV (ARMD, central retinal vein occlusion, etc.) should be borne in mind6,7,8.
Will treatment inevitably be of benefit?
An important aspect here is the NNT = number of patients needed to be treated in order to prevent a negative outcome in ONE patient. Ideally, this number should be low. In the CNTGS,9,10 it was necessary to treat 17 patients (30% IOP reduction) in order to prevent progression in ONE patient. Seven of these 17 patients will develop cataract. Is it worth treating 17 patients when only ONE patient will be protected and seven will develop cataract?11
CCT
Corrected tonometric values are advisable when IOP measurements are high
and treatment is being considered. The true value of CCT measurements is
to discover falsely high readings (with all the consequences) caused by
thick corneas in OHT or falsely low readings where glaucomatous optic neuropathy
exists at presumably normal IOP. The relative importance of CCT measurements
should be seen in the light of the well-known fact that inter-individual
variation of IOP measurements may be up to 4 mmHg.
For the management of glaucoma, CCT measurements are of limited value since
this is based on the follow-up of disc/RNFL and VF.
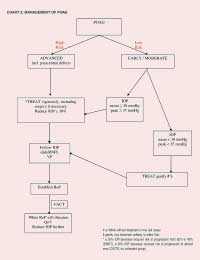
POAG: Individualized Treatment (Chart 2)
Let the diagnosis be based on firm evidence. Labelling a patient as having
glaucoma may in itself reduce QoL.12
The mean age of most patients detected is just under 70 years (EMGT).13
Average life expectancy is about ten years. Mean progression RATE (MD in
dB/month) is:
| Rate of Progression MD in dB / month |
| - T | + T |
| dB/M | 0.05 | 0.03 | dB/Y | 0.6 | 0.36 | dB/10 Y | 6.0 | 3.60 |
Advanced Glaucoma = -16 dB Advanced defects (excluded from the EMGT) were defined as MD of minus
16 dB or less.
Is greater IOP reduction inevitably better?Quite a few studies have suggested this. The most frequently quoted paper is AGIS-715: although these results are rather suggestive, they are not the result of a specifically-designed intent-to-treat study. However, they do confirm our clinical impressions. EMGT-2 comes close to convincing us, with the finding that 10% protection is obtained for every ONE mmHg IOP decrease. CIGTS16 had a target IOP reduction of 35%: to reach that target, 22% of patients needed ALT plus medical treatment, while 8% needed additional surgery. Assuming we accept that medical treatment and ALT may be relatively benign in terms of QoL, over 90% of patients can reach a substantial reduction of IOP with that combination. Greater IOP reduction (vigorous treatment) may include surgical reduction of IOP. Both medical and surgical treatment (much more) may cause cataract. When vigorous IOP reduction is needed, the risk of cataract is not considered to be a prohibitive side-effect, given the results of modern cataract surgery. There was no difference in the QoL of the medically and surgically treated groups in CIGTS17. The possible vision-threatening side-effects of anti-metabolite-assisted surgery are well-known. Benefits in terms of avoidance of further QoV decrease should be weighed against the risk of decrease of QoV/QoL by these side-effects. References
|

